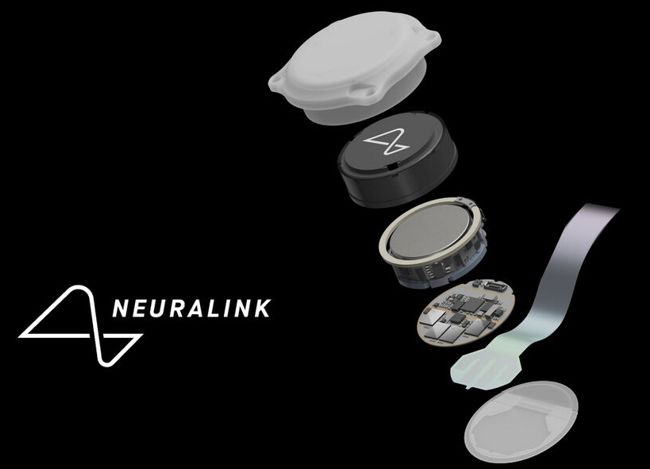
Image credit: Neuralink
Elon Musk posted on X that Neuralink conducted the first human implant of its brain-computer interface:
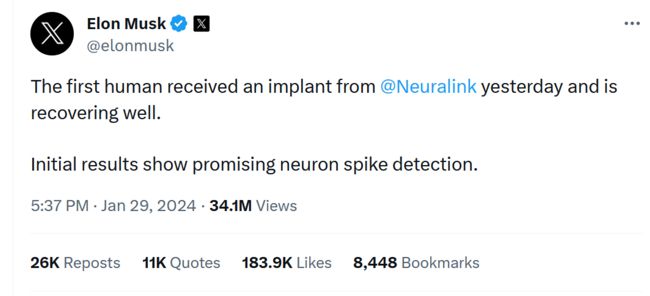
Image Capture from X
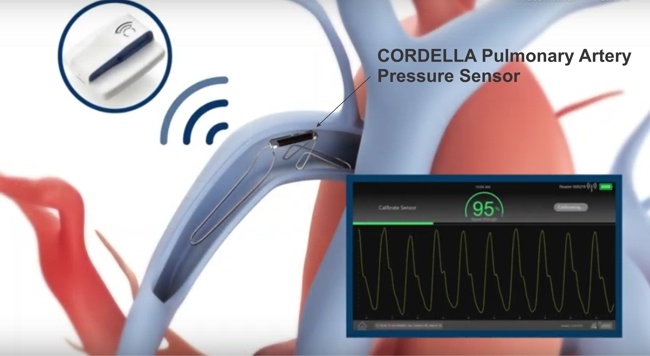
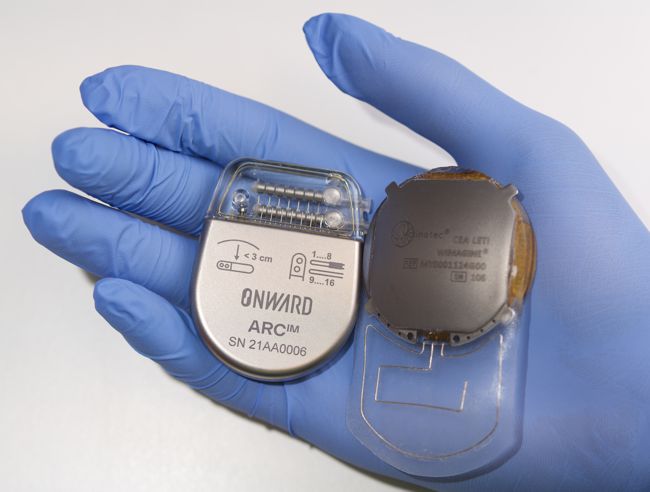
Details are scant at this time, but Neuralink announced back in September ’23 that they had started recruiting subjects with quadriplegia due to cervical spinal cord injury or amyotrophic lateral sclerosis (ALS) for their PRIME Study (short for Precise Robotically Implanted Brain-Computer Interface), which is an IDE trial for their fully-implantable, wireless brain-computer interface (N1) and surgical robot (R1).