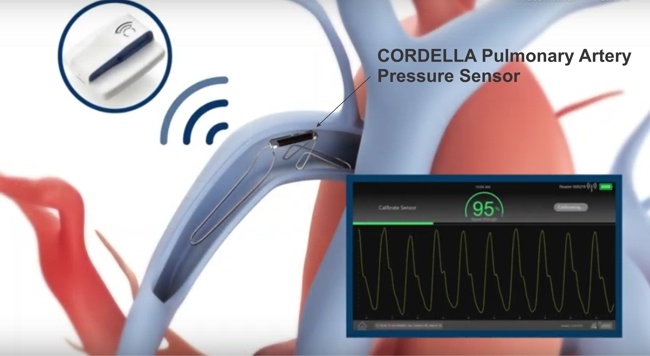
Endotronix announced that it received FDA’s PMA approval for its Cordella™ Pulmonary Artery (PA) Sensor System for the treatment of NYHA class III heart failure patients. According to Endotronix, “The Cordella platform is the first and only PA pressure-guided platform to offer comprehensive patient management using daily PA pressure and vital signs from home to guide therapeutic management and improve patient outcomes.”
According to the announcement:
“Cordella is a proactive HF management platform that delivers daily PA pressure and other vital data via an implantable sensor and user-friendly, non-invasive health tools, respectively, to a managing HF clinician for remote patient care. This information guides clinical decision-making and medication dosing while enhancing the adoption of guideline-directed medical therapy (GDMT) to reduce congestion and improve outcomes. Regulatory approval was based on the PROACTIVE-HF trial, which demonstrated a markedly low 0.159 rate of heart failure hospitalization and all-cause mortality at 6 months.”