
Image Credit: CorTec
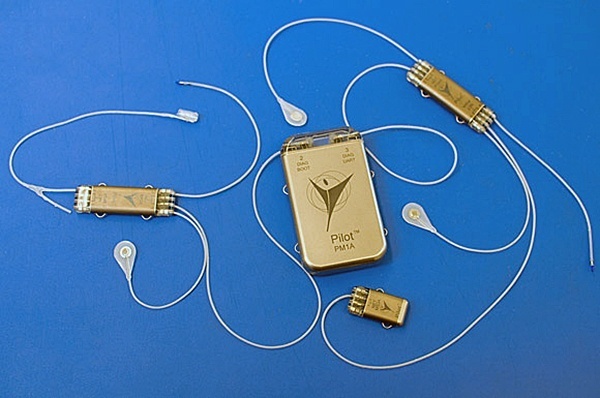
CorTec GmbH announced that it had entered into a strategic partnership with Heraeus Medevio, based in Minneapolis, USA. The venture aims to support customers in the neuromodulation sector, from concept and small series production (CorTec) to large series production (Medevio) of nerve cuff and paddle electrodes.
According to the announcement, Medevio is expected to begin high-volume manufacturing of nerve cuff and paddle electrodes based on CorTec’s proprietary laser-micromachining technology by the end of 2025.

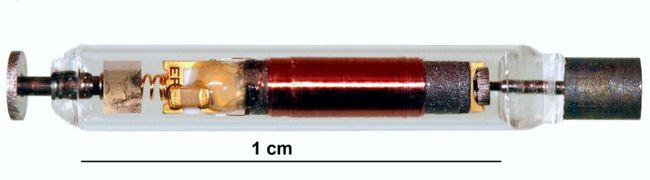
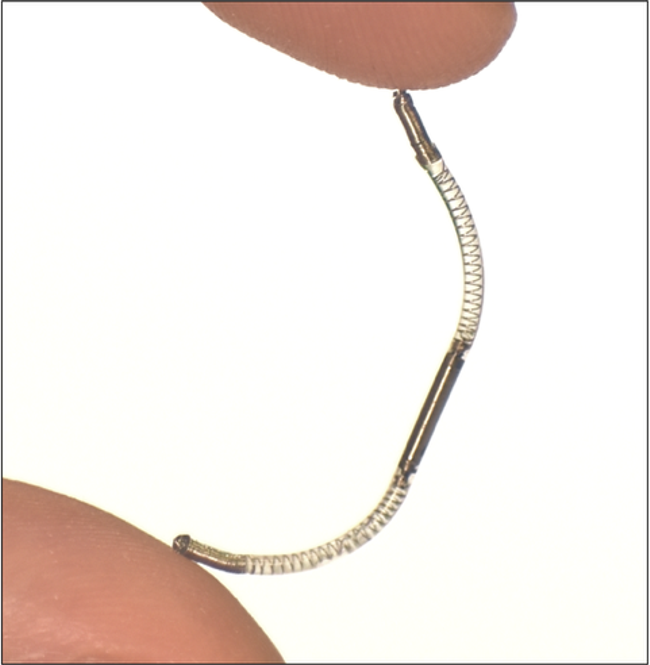
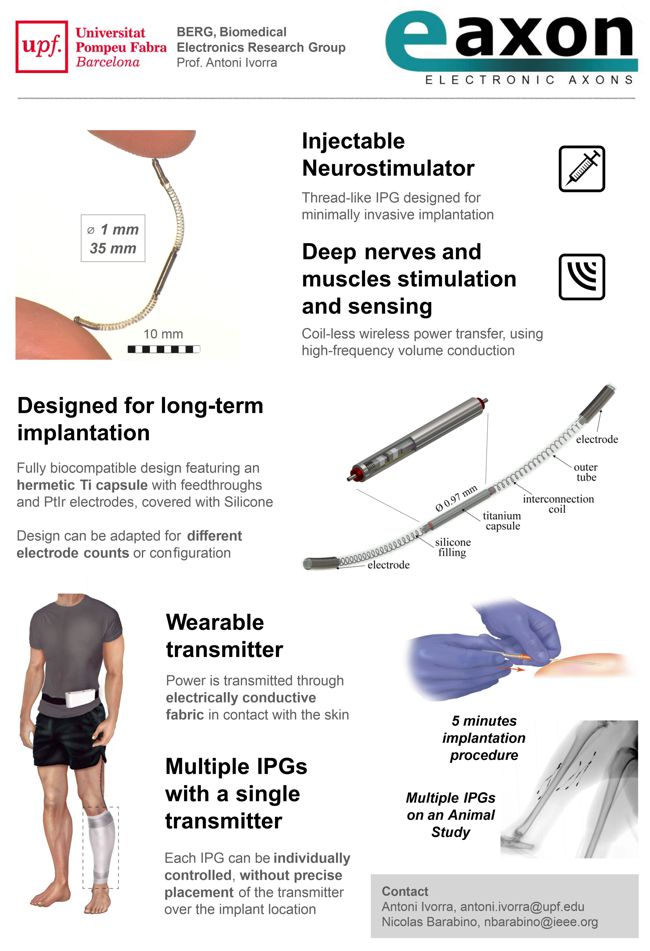


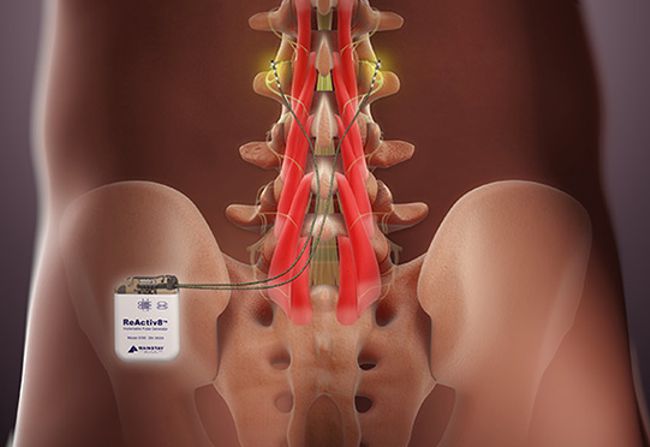

![logo_mainstay1[1]](https://www.implantable-device.com/wp-content/uploads/2013/06/logo_mainstay11.jpg)


 BioControl Medical, Ltd. was founded in 1999 by
BioControl Medical, Ltd. was founded in 1999 by 