Image Caption: Newronika
Newronika, was founded in 2016 to develop adaptive deep-brain stimulation technology. The Company is the spin-off company of two Italian research institutions, the Fondazione IRCCS Ca’ Granda Ospedale Maggiore Policlinico Hospital and the University of Milan.
Earlier this week Newronika announced that it received CE Mark approval for its AlphaDBS device for the treatment of Parkinson’s disease (PD).
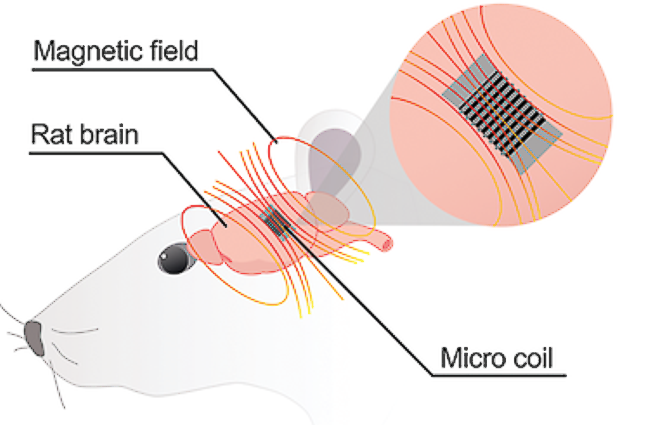
Unlike open-loop DBS stimulators, Newronika’s AlphaDBS senses local field potentials (LFPs) to adapt the stimulation in real-time to optimize the patient’s clinical condition.
The CE Mark approval was supported by clinical data showing that patients experienced an extended period of time without symptoms or side effects as compared to conventional, open-loop DBS. Patients reported enhanced quality of life and a preference for the adaptive stimulation mode.
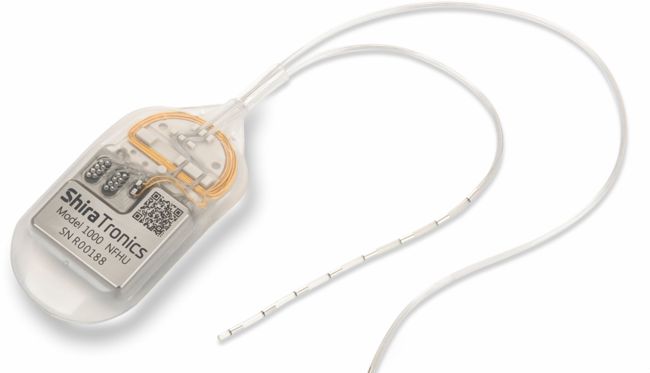
The AlphaDBS rechargeable IPG has 2 channels for recording LFPs, 1 week of memory capacity for biomarker trend and spectrogram, and 16 current-independent channels for DBS.