On February 4, 2013, after more than 20 years of research and development, Second Sight Medical Products, Inc., announced that its Argus® II Retinal Prosthesis System (“Argus II”) has received U.S. market approval from the Food and Drug Administration (FDA) to treat individuals with late stage retinitis pigmentosa (RP). This announcement follows receipt of the European approval in 2011, and a unanimous recommendation by the FDA’s Ophthalmic Devices Advisory Panel in September 2012 that this revolutionary product be made available to treat this patient population in the U.S.
Category Archives: Neural Stimulation
AIMDs for the stimulation of the nervous system
Medtronic Introduces First Neuromodulation Systems Compatible with Full-Body MRI
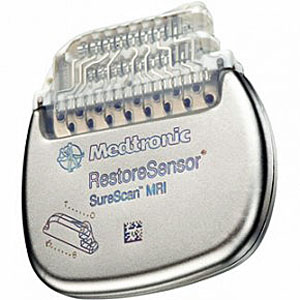
Medtronic has introduced in Europe the first and only implantable neurostimulation systems indicated for use in the treatment of chronic back and/or leg pain that are designed for full-body Magnetic Resonance Imaging (MRI) scans under specific conditions. Medtronic SureScan neurostimulation systems include enhancements to existing devices as well as specially designed leads to reduce or eliminate the hazards produced by the MRI environment. The devices also include a proprietary SureScan programming feature, which sets the device into an appropriate mode for the MRI environment.
SetPoint Medical’s Vagus Nerve Stimulation for Treatment of Systemic Inflammation
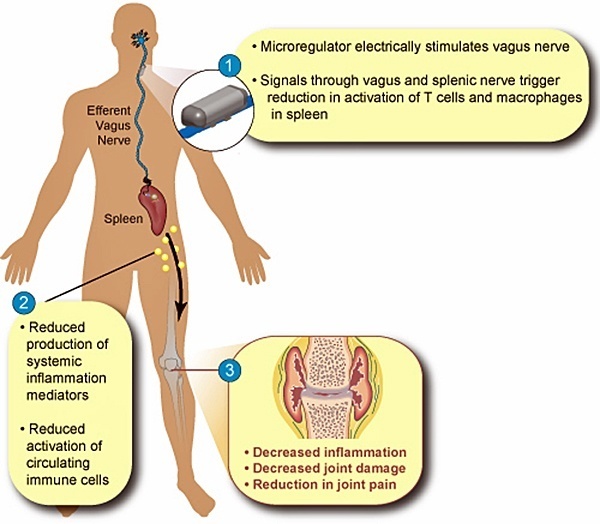
SetPoint Medical, headquartered in Valencia, California, is developing neuromodulation therapies for patients with inflammatory autoimmune diseases, such as rheumatoid arthritis (RA), inflammatory bowel disease (IBD), psoriasis, diabetes, heart disease, and multiple sclerosis. SetPoint’s proprietary neuromodulation platform consists of an implantable “microregulator”, wireless charger and iPad prescription pad application.
Functional Neuromodulation Ltd. Starts Study using DBS of the Fornix (DBS-f) for Mild Alzheimer’s
 Toronto-based Functional Neuromodulation announced that it implanted the first U.S. Alzheimer’s patient in the “ADvance Study” with a deep brain stimulation (DBS) system meant to improve cognitive performance. ADvance will evaluate the safety and potential clinical benefit of DBS of the fornix (DBS-f), a major inflow and output pathway in the brain’s memory circuit, for patients with mild Alzheimer’s. The ADvance Study is being conducted using Medtronic Activa DBS IPGs.
Toronto-based Functional Neuromodulation announced that it implanted the first U.S. Alzheimer’s patient in the “ADvance Study” with a deep brain stimulation (DBS) system meant to improve cognitive performance. ADvance will evaluate the safety and potential clinical benefit of DBS of the fornix (DBS-f), a major inflow and output pathway in the brain’s memory circuit, for patients with mild Alzheimer’s. The ADvance Study is being conducted using Medtronic Activa DBS IPGs.
While DBS has been an effective treatment for movement disorders for more than 15 years, it was only recently that this approach was first applied to Alzheimer’s. Dr. Andres Lozano, a neurosurgeon at University of Toronto and Scientific Founder of Functional Neuromodulation, originated the concept of treating memory disorders using deep brain stimulation (DBS) while treating a patient suffering from morbid obesity. In this patient, DBS stimulation of the hypothalamus and fornix was associated with an unexpected observed improvement in the patient’s memory.
Boston Scientific’s Precision Spectra™ SCS with 32 Contacts and 32 Dedicated Power Sources Receives CE Mark

Boston Scientific Received European Regulatory Approval For New Precision Spectra™ Spinal Cord Stimulator System. It is the first and so-far only SCS system with 32 contacts and 32 dedicated power sources designed to provide pain relief to a broad spectrum of chronic pain patients.
Boston Scientific Receives CE Mark of Vercise™ Deep Brain Stimulation System
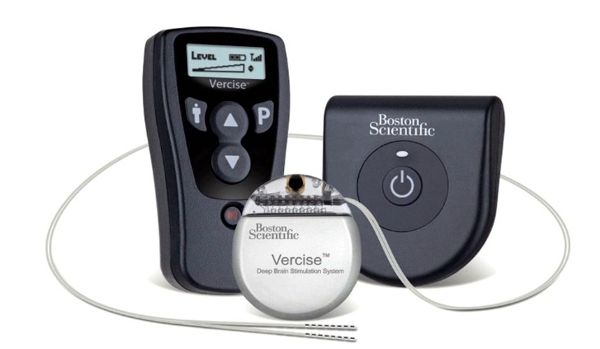
Image Credit: Boston Scientific
Boston Scientific Corporation received CE Mark approval for use of its Vercise™ Deep Brain Stimulation (DBS) System for the treatment of Parkinson’s disease. The Vercise DBS System is the first and only commercially available DBS system to incorporate multiple independent current control, which is designed to selectively stimulate targeted areas in the brain. This system is an innovative technology that is designed to provide physicians fine control of stimulation.
First Human Use of 24-Electrode Retinal Implant by Bionic Vision Australia
Bionic Vision Australia researchers announced on August 30, 2012 that they successfully performed the first implantation of an early prototype bionic eye with 24 electrodes.
According to the press release:
St. Jude Medical Receives CE Mark Approval of Eon Mini to Treat Chronic Migraine
 St. Jude Medical announced it has received European CE Mark approval of its Eon™ family of neurostimulators for treating patients with intractable chronic migraine.
St. Jude Medical announced it has received European CE Mark approval of its Eon™ family of neurostimulators for treating patients with intractable chronic migraine.
According to the press release:
“Intractable chronic migraine is one of the most difficult-to-treat headache disorders,” said Professor Gennaro Bussone, M.D., head of the Neurological Department at Istituto Besta in Milan Italy. “By definition, people living with this condition are spending half their month living with debilitating headaches. This therapy expands our options in helping manage patients who suffer with disabling chronic migraine symptoms.”
Nevro Receives FDA Approval to Initiate Trial of its High-Frequency Spinal Cord Stimulation Therapy

Image Source: Nevro's Website
Nevro Corp announced that FDA has granted approval for initiation of its SENZA-RCT study, a U.S. prospective, randomized, controlled pivotal clinical trial evaluating the safety and efficacy of Nevro’s high-frequency spinal cord stimulation system for the treatment of chronic pain.
EnteroMedics Reports $123k Revenue from Sales of its Maestro RC IPG for Treatment of Obesity
 Today EnteroMedics recorded revenue for the first time since it was incorporated nearly eight years ago. The company reported revenue of about $123,000 in the first quarter of the year from the sale of its Maestro RC implantable vagus nerve stimulation system for treating obesity. Revenue was generated through sales by its distribution partner in Australia.
Today EnteroMedics recorded revenue for the first time since it was incorporated nearly eight years ago. The company reported revenue of about $123,000 in the first quarter of the year from the sale of its Maestro RC implantable vagus nerve stimulation system for treating obesity. Revenue was generated through sales by its distribution partner in Australia.
This is an important step for an implantable device company that faced very tough times in 2009 after its US clinical trial failed to meet a critical effectiveness goal. EnteroMedics is currently conducting a pivotal trial that is expected to end in Q4 2012. EnteroMedics expects to file a premarket approval application with the FDA in the first half of 2013 if the data from the trial is positive.
Monash University in Australia Starts Test of Direct-to-Brain Visual Prosthesis Chips
Image Credit: Monash Vision Group, Monash University, Australia
Engineers from the Monash Vision Group (MVG) have begun trialling the ASICs for a direct-to-brain visual prosthesis that is expected to enter human clinical trials in 2014.
The prosthesis will consist of a tiny camera mounted into a pair of glasses, which acts as the retina; a pocket processor, which takes the electronic information from the camera and converts it into signals enabling the brain to build up a visual construct; and cortical implants of several tiles which will be the portal for the stimulation of the visual cortex. Continue reading
John Hopkins Researcher Develops New Early-Warning Seizures Detector with Low False-Positive Rate
 Johns Hopkins’ Sridevi V. Sarma, an assistant professor of biomedical engineering, has devised new seizure detection software that, in early testing, significantly cuts the number of unneeded brain-stimulation therapy that an epilepsy patient would receive.
Johns Hopkins’ Sridevi V. Sarma, an assistant professor of biomedical engineering, has devised new seizure detection software that, in early testing, significantly cuts the number of unneeded brain-stimulation therapy that an epilepsy patient would receive.
According to Sarma, “These devices use algorithms—a series of mathematical steps—to figure out when to administer the treatment,” Sarma said. “They’re very good at detecting when a seizure is about to happen, but they also produce lots of false positives, sometimes hundreds in one day. If you introduce electric current to the brain too often, we don’t know what the health impacts might be. Also, too many false alarms can shorten the life of the battery that powers the device, which must be replaced surgically.” Continue reading
Neuromed’s TIME Battery- and RF-Powered Totally Implantable Multichannel Spinal Cord Stimulator (ca. 1988)

Neuromed TIME IPG on loan from Daniel Villamil's collection.
Neuromed was formed in 1980 with an initial capitalization of $150,000 by Bill Borkan through money obtained when Borkan`s parents took out a second mortgage on their home. Borkan’s desire to help his sister, Jennie, a cerebral palsy patient, got him started in neurostimulation technology. In the next few years, Neuromed developed and marketed a RF-powered implantable spinal cord stimulator, along with its external radio frequency transmitter.
Throughout the 1980s, development of more advanced devices was ongoing at Neuromed. My friend Daniel Villamil from CCC Medical has in his collection one of these more modern units, which he lent to me for photographing. The “Total Implantable Multichannel Electronics” (TIME) spinal cord stimulator shown in this picture went into clinical trials around 1988. This was a device that was internally powered by its own battery. However, it could also be RF-powered after the eventual battery failure. Continue reading
St. Jude’s (ANS) Rechargeable Spinal Cord Stimulators Eon and Eon Mini

In 2005, St. Jude Medical purchased Advanced Neuromodulation Systems (ANS) in Plano, Texas. ANS had developed a number of spinal cord stimulation IPGs that were either externally powered via inductive link, internally powered by a primary cell, or internally powered by a transcutaneously rechargeable lithium-ion cell.
Today, the most popular St. Jude spinal cord stimulators are the rechargeable 42 cc Eon and 18 cc Eon mini neurostimulators.
They are constant-current devices with a rated longevity of 10 years. Current through up to 16 electrodes is programmable between 0-25.5 mA with a pulse width of 50-500 µs and a frequency between 2-1200 Hz. Continue reading
St. Jude’s DBS Study Confirms Benefit of Constant Current System for Parkinson’s Disease

- Image Credit: St. Jude Medical
Today St. Jude announced that its first controlled study of Deep Brain Stimulation (DBS) confirms benefit of constant current system for patients with Parkinson’s Disease.
Results were published online today by The Lancet Neurology journal. The aim of the study was to evaluate the Libra(TM) and LibraXP(TM) DBS constant current systems to determine the devices’ safety and effectiveness in managing the symptoms of PD. Continue reading

