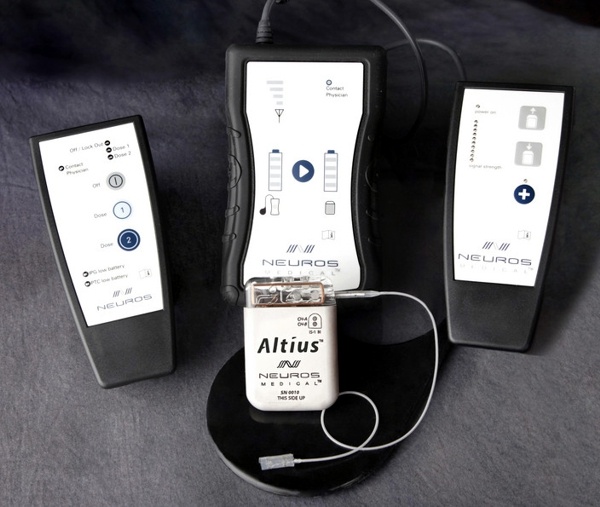
Neuros Medical, a Cleveland, Ohio based neuromodulation company, developed the Altius implantable system to deliver Electrical Nerve Block therapy for the treatment of chronic pain in a variety of applications including neuroma/residual limb pain, chronic post surgical pain, and chronic migraine.

![SynchroMed-II[1]](https://www.implantable-device.com/wp-content/uploads/2013/06/SynchroMed-II1-300x272.jpg)
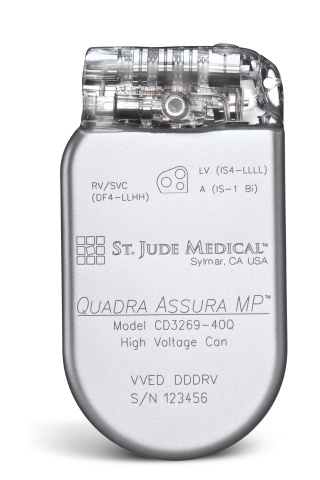 St. Jude Medical today announced CE Mark approval of its next-generation quadripolar device, the Quadra Assura MP™ cardiac resynchronization therapy defibrillator (CRT-D). The device features MultiPoint™ Pacing (MPP) technology that enables physicians to pace multiple locations on the left side of the heart. This gives the clinician more choices to best optimize cardiac resynchronization therapy (CRT) pacing to meet individual patient needs.
St. Jude Medical today announced CE Mark approval of its next-generation quadripolar device, the Quadra Assura MP™ cardiac resynchronization therapy defibrillator (CRT-D). The device features MultiPoint™ Pacing (MPP) technology that enables physicians to pace multiple locations on the left side of the heart. This gives the clinician more choices to best optimize cardiac resynchronization therapy (CRT) pacing to meet individual patient needs. Sorin announced today CE mark approval and the European commercial launch of the REPLY ™ 200 family of pacemakers featuring Sleep Apnea Monitoring (SAM). According to the press release:
Sorin announced today CE mark approval and the European commercial launch of the REPLY ™ 200 family of pacemakers featuring Sleep Apnea Monitoring (SAM). According to the press release:
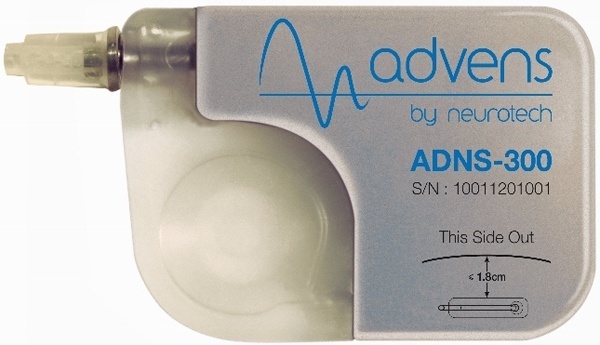

 St. Jude Medical and privately-held Spinal Modulation, Inc., today announced that they have entered into a series of agreements under which St. Jude Medical made a $40 million equity investment in Spinal Modulation, a company that has developed an innovative neuromodulation therapy that provides a new pain management option for patients with chronic, intractable pain.
St. Jude Medical and privately-held Spinal Modulation, Inc., today announced that they have entered into a series of agreements under which St. Jude Medical made a $40 million equity investment in Spinal Modulation, a company that has developed an innovative neuromodulation therapy that provides a new pain management option for patients with chronic, intractable pain.
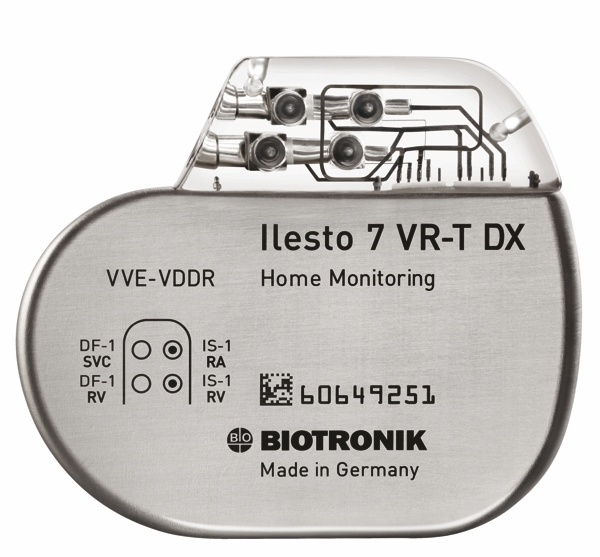
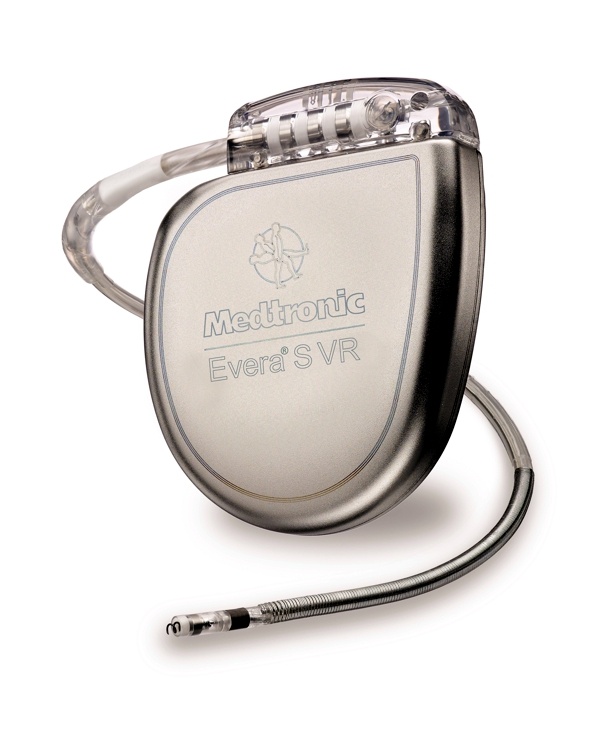
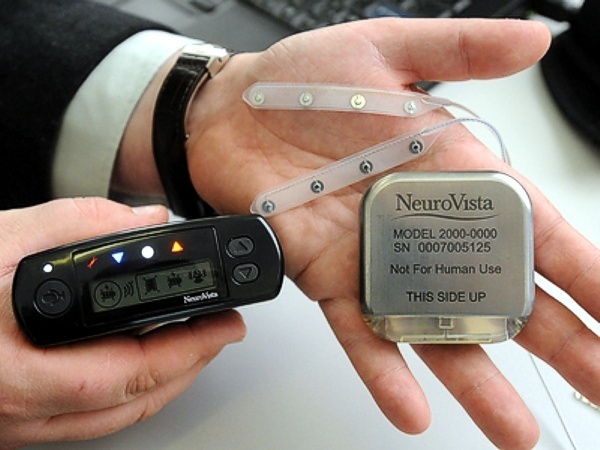
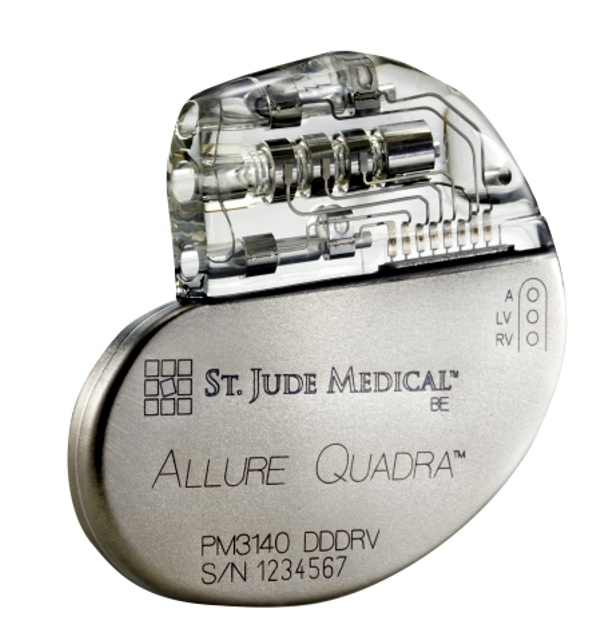 St. Jude Medical today announced CE Mark approval and European launch of its Allure Quadra™ Cardiac Resynchronization Therapy Pacemaker (CRT-P), which brings the quadripolar lead technology to the pacemaker market for the first time. According to the press release:
St. Jude Medical today announced CE Mark approval and European launch of its Allure Quadra™ Cardiac Resynchronization Therapy Pacemaker (CRT-P), which brings the quadripolar lead technology to the pacemaker market for the first time. According to the press release:
