
Image Credit: St. Jude Medical
AIMDs classified according to general type of therapy

Image Credit: St. Jude Medical
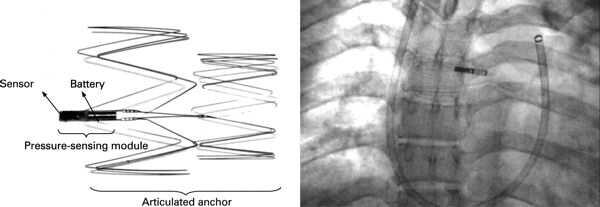
Remon Medical Technologies, Ltd. was founded in 1997 in Caesarea, Israel to develop implantable, wireless pressure sensors.
Remon developed an implantable hemodynamic monitor, which allowed on-demand, non-invasive, leadless self-monitoring of pulmonary artery pressure by the patient at home. ImPressure devices were placed in the pulmonary artery, and transmitted pressure readings to a hand-held monitor. It was hoped that the system would provide early warning of the need for treatment, avoiding hospitalization and deterioration in the patient’s condition. Continue reading→
 Sensors for Medicine and Science, Inc. (SMSI) of Germantown, MD was founded in 1997 to develop chemical sensing technologies based on fluorescence sensing.
Sensors for Medicine and Science, Inc. (SMSI) of Germantown, MD was founded in 1997 to develop chemical sensing technologies based on fluorescence sensing.
SMSI® is now developing an implantable glucose sensor that is designed to automatically measure interstitial glucose every few minutes. The sensor implant communicates wirelessly with a small external reader, allowing it to track the rate of change of glucose levels and warn the user of impending hypo- or hyperglycemia. According to SMSI, the target operational life of the sensor implant will be 6-12 months, after which it would be replaced. Continue reading→
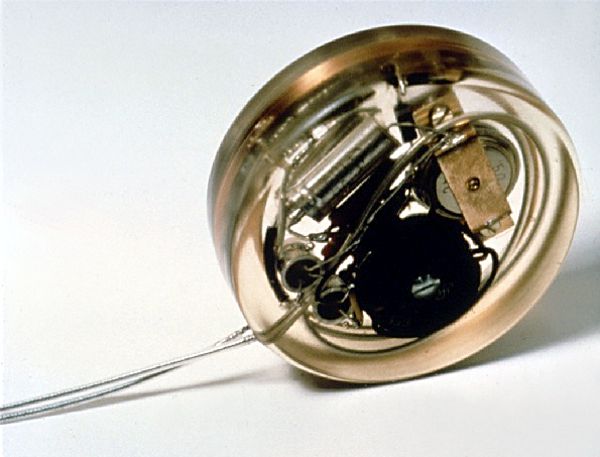
This is a picture of the first pacemaker to be implanted in a human patient. It was developed by Dr. Rune Elmqvist (1906–1996), a physician by training, but working for the Swedish company Elema-Schonander as an engineer. Dr. Elmqvist developed the device in cooperation of Åke Senning, senior physician and cardiac surgeon at the Karolinska University Hospital in Solna, Sweden. Continue reading→

Image Credit: MicroCHIPS
MicroCHIPS was founded in 1999 as an MIT spinoff to develop implantable sensors and drug-delivery devices.
MicroCHIPS’ drug-delivery technology is based on proprietary reservoir arrays that are used to store potent drugs within the body for long periods of time. Individual device reservoirs can be opened on demand or on a predetermined schedule to precisely control drug release or sensor activation. Continue reading→

Neuromed TIME IPG on loan from Daniel Villamil's collection.
Neuromed was formed in 1980 with an initial capitalization of $150,000 by Bill Borkan through money obtained when Borkan`s parents took out a second mortgage on their home. Borkan’s desire to help his sister, Jennie, a cerebral palsy patient, got him started in neurostimulation technology. In the next few years, Neuromed developed and marketed a RF-powered implantable spinal cord stimulator, along with its external radio frequency transmitter.
Throughout the 1980s, development of more advanced devices was ongoing at Neuromed. My friend Daniel Villamil from CCC Medical has in his collection one of these more modern units, which he lent to me for photographing. The “Total Implantable Multichannel Electronics” (TIME) spinal cord stimulator shown in this picture went into clinical trials around 1988. This was a device that was internally powered by its own battery. However, it could also be RF-powered after the eventual battery failure. Continue reading→

This is a hack that combines three of my favorite passions: pacemakers, photography, and coffee!
I took this photograph by feeding the output of an infrared barrier to the atrium input of an old DDD pacemaker, setting an appropriate AV delay, and using the ventricular output to trigger a camera flash (via a optoisolator). In a darkened room, I opened my camera’s shutter for 2 seconds. I then let one drop of milk fall through the infrared barrier, starting the AV delay in VAT mode. The flash then fires as the drop enters the coffee in the cup, freezing the action. Continue reading→

In 2005, St. Jude Medical purchased Advanced Neuromodulation Systems (ANS) in Plano, Texas. ANS had developed a number of spinal cord stimulation IPGs that were either externally powered via inductive link, internally powered by a primary cell, or internally powered by a transcutaneously rechargeable lithium-ion cell.
Today, the most popular St. Jude spinal cord stimulators are the rechargeable 42 cc Eon and 18 cc Eon mini neurostimulators.
They are constant-current devices with a rated longevity of 10 years. Current through up to 16 electrodes is programmable between 0-25.5 mA with a pulse width of 50-500 µs and a frequency between 2-1200 Hz. Continue reading→

In 1973, former Medtronic sales representative Albert Beutel founded Intermedics in Freeport, TX. The first product was a small, mercury-cell-powered pacemaker. In 1974 Intermedics introduced a lithium-powered version, and in 1976 it introduced InterLith which was hermetically sealed, and weighed just 65 grams. At the time, InterLith’s size was a breakthrough, and became a very popular device, solidifying Intermedics’ position in the industry.

Some time ago, my friend and colleague Paul Spehr gave me a copy of Arco Medical’s product catalog. I scanned the original datasheets for Arco Medical’s nuclear fixed-rate and demand pacemakers models NU-5 and NU-6 and posted them here in pdf format: Arco_Nuclear_Datasheets
Click here for a color picture and more information on Arco Medical’s nuclear pacemakers.

Today St. Jude announced that its first controlled study of Deep Brain Stimulation (DBS) confirms benefit of constant current system for patients with Parkinson’s Disease.
Results were published online today by The Lancet Neurology journal. The aim of the study was to evaluate the Libra(TM) and LibraXP(TM) DBS constant current systems to determine the devices’ safety and effectiveness in managing the symptoms of PD. Continue reading→

The Cardio Care II Pacemaker was American Optical’s second implantable device. It was an improved version of the Cardio Care pacemaker. Besides improvements to the circuitry, the circuit board was enclosed separately inside a hermetic can within the epoxy encapsulation. Continue reading→
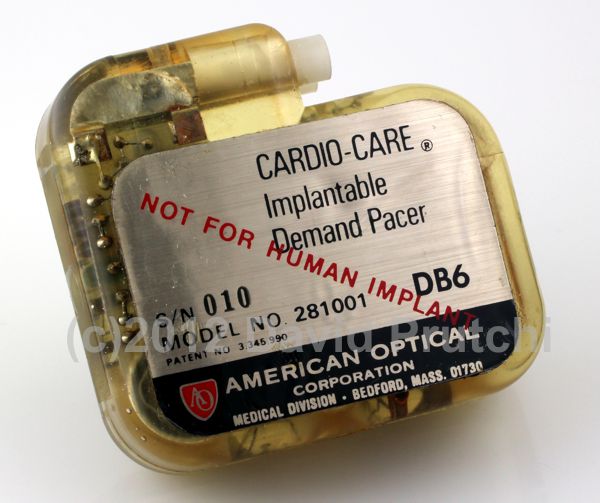
Barouh Berkovits at American Optical Co of Boston, MA designed the first “Demand Pacemaker” – what we now know as a VVI pacemaker. The Cardio-Care Demand Pacemaker, introduced in 1968, was American Optical’s first implantable device.
From Kirk Jeffrey’s Machines in our Hearts(2001):
“Berkovits in 1963 designed a sensing capability into the pacemaker. His invention behaved exactly like an asynchronous pacer until it detected a naturally occurring R wave, the indication of a ventricular contraction. This event would reset the timing circuit of the pacemaker, and the countdown to the next stimulus would begin anew. Thus the pacer stimulated the heart only when the ventricles failed to contract. It worked only ‘‘on demand.’’ As an added benefit, noncompetitive pacing extended the life of the battery. Continue reading→

Biotectix of Ann Arbor, MI recently contacted me to let me know of new conductive polymer materials that they are developing to enhance the performance of next-gen implantable stimulation and sensing devices.
Indeed, their materials sound very promising. According to Biotectix, their electrode coatings and device components are made from proprietary conducting polymers that provide intimate, long-term electrical and biological connections between implantable electrodes and the target tissue. They offer the conductivity and stability of metals at a low-cost with the ease of processing and biological functionality of polymers. Continue reading→

I took this picture a very long time ago at the office of one of my implanter friends in Europe. Ever since then, I’ve tried to find out about “Digikon,” but have had no luck so far. All that I have been able to find from the St. Jude legacy device database is that Digikon had produced a number of pacemaker models, including the one shown in this picture.