The 2015 “Made in China 2025” and subsequent domestic development initiatives have significantly benefited Chinese medical device manufacturers. The 2015 plan prioritized the expansion of high-tech device production with the goal of supplying 70% of China’s device market by 2025. More recently, in 2021, the Chinese government issued the 14th five-year plan (2021–25) that aims at making at least six Chinese companies reach the top 50 revenue-making medical device companies globally.
I was recently chatting with Victor Pikov at the First AIMD Workshop, and he mentioned that in the last couple of years the number of Chinese AIMD manufacturers have grown from 5 to over 10. I was really surprised about the progress that China has made in the AIMD field, so this blog expands on the list of Chinese AIMD companies that Victor compiled and kindly shared with me. Continue reading

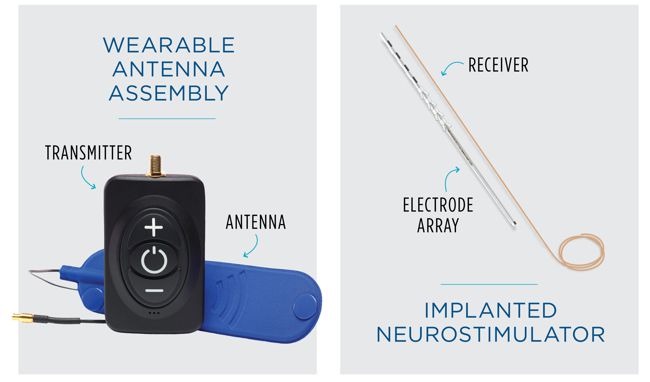
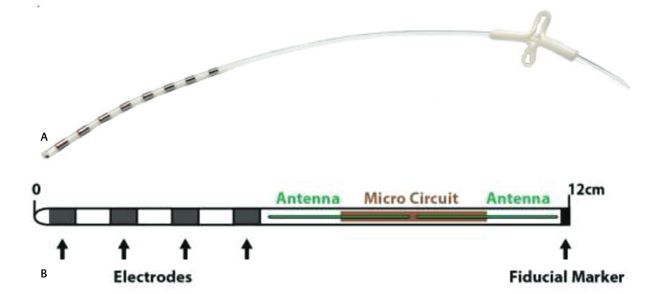


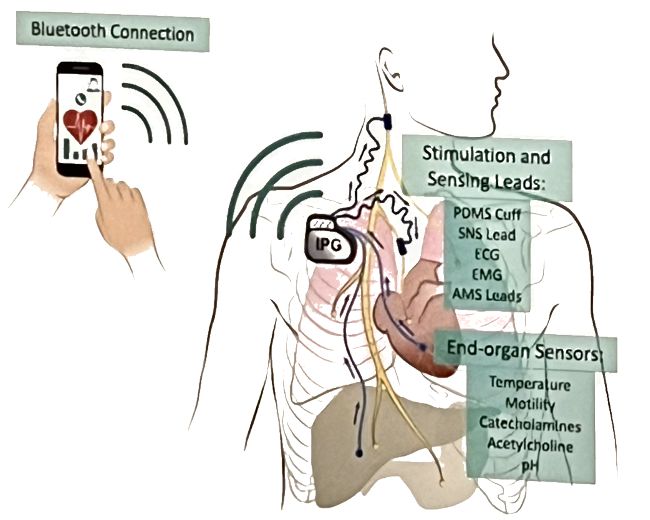
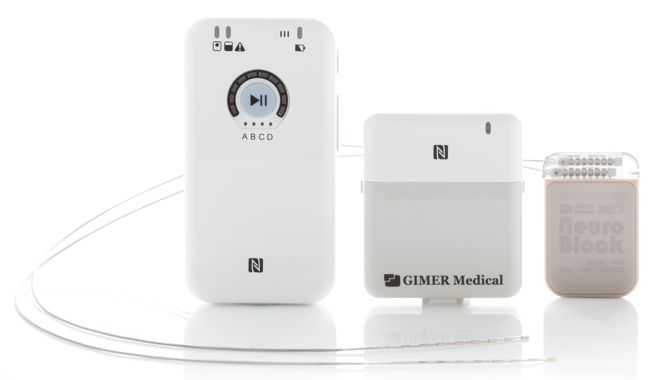
 Impulse Dynamics, the company where I’m CTO and Executive VP,
Impulse Dynamics, the company where I’m CTO and Executive VP,