From: Lorenz, Carrie & Sandoval, Wendolyn & Mortellaro, Mark. (2018). Interference Assessment of Various Endogenous and Exogenous Substances on the Performance of the Eversense Long-Term Implantable Continuous Glucose Monitoring System. Diabetes Technology & Therapeutics. 20. 10.1089/dia.2018.0028.
Senseonics in Germantown, MD announced that its Eversense® Implantable Continuous Glucose Monitoring System has received the iCGM designation by the FDA. iCGM status indicates that Senseonics’ Eversense iCGM product can integrate with compatible medical devices, including insulin pumps as part of an automated insulin delivery (AID) system.
According to the announcement:
“As the first fully implantable device in the category, Eversense has been authorized to be marketed as an iCGM through the FDA’s De Novo pathway, by establishing the special controls that will serve as a predicate device for 510(k) submissions in the future for devices of the same type.
… The companies plan to advance partnership discussions with various pump manufacturers, with plans to offer people who choose to integrate their diabetes devices a new interoperable CGM option that is exceptionally well suited for AID systems. This is because Eversense addresses common limitations of AID systems outlined in the 2022 Consensus Report of the Joint Diabetes Technology Working Group of the European Association for the Study of Diabetes and the American Diabetes Association.”
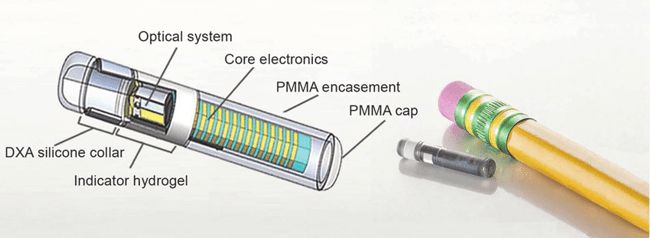
The Eversense subcutaneously implantable glucose sensor has been validated for up to 180-day implant periods. Unlike other CGM systems that use
electrochemical- and enzymatic- (i.e., glucose oxidase and glucose dehydrogenase) based methods to measure glucose concentrations, the Eversense CGM sensor uses an abiotic (non–enzyme based), fluorescent glucose-indicating polymer to measure glucose.