EnteroMedics reported that it has submitted its pre-market approval application for FDA review of its Maestro Rechargeable System’s VBLOC vagal blocking therapy as a treatment for obesity
EnteroMedics reported that it has submitted its pre-market approval application for FDA review of its Maestro Rechargeable System’s VBLOC vagal blocking therapy as a treatment for obesity
This is an important step for an implantable device company that faced very tough times in 2009 after its US clinical trial failed to meet a critical effectiveness goal. EnteroMedics conducted a pivotal trial, and believes that the data are positive, leading it to submit the PMA application.
According to the press release, EnteroMedics president & CEO Mark Knudson said that “the Maestro System is a unique, neuroscience-based approach to the treatment of this epidemic disease, one which offers the potential to fill a significant gap in the obesity treatment spectrum. In the hundreds of patients treated to date, in addition to showing clinically meaningful weight loss, VBLOC Therapy has demonstrated an excellent benefit-to-risk profile; a criterion identified by the Agency as central to the review and approvability of new obesity treatment devices.”




![logo_mainstay1[1]](https://www.implantable-device.com/wp-content/uploads/2013/06/logo_mainstay11.jpg)
![SynchroMed-II[1]](https://www.implantable-device.com/wp-content/uploads/2013/06/SynchroMed-II1-300x272.jpg)
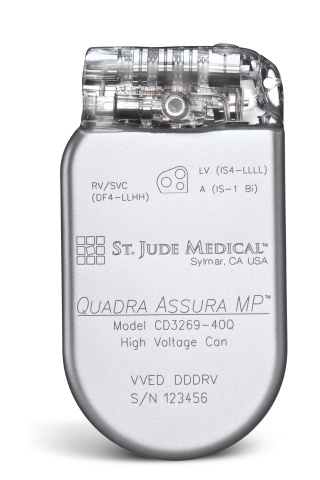 St. Jude Medical today announced CE Mark approval of its next-generation quadripolar device, the Quadra Assura MP™ cardiac resynchronization therapy defibrillator (CRT-D). The device features MultiPoint™ Pacing (MPP) technology that enables physicians to pace multiple locations on the left side of the heart. This gives the clinician more choices to best optimize cardiac resynchronization therapy (CRT) pacing to meet individual patient needs.
St. Jude Medical today announced CE Mark approval of its next-generation quadripolar device, the Quadra Assura MP™ cardiac resynchronization therapy defibrillator (CRT-D). The device features MultiPoint™ Pacing (MPP) technology that enables physicians to pace multiple locations on the left side of the heart. This gives the clinician more choices to best optimize cardiac resynchronization therapy (CRT) pacing to meet individual patient needs. Sorin announced today CE mark approval and the European commercial launch of the REPLY ™ 200 family of pacemakers featuring Sleep Apnea Monitoring (SAM). According to the press release:
Sorin announced today CE mark approval and the European commercial launch of the REPLY ™ 200 family of pacemakers featuring Sleep Apnea Monitoring (SAM). According to the press release:
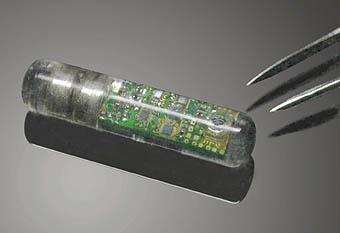

 FDA has published a draft of the guidance document that it has developed to assist industry by identifying issues related to cybersecurity that manufacturers should consider in preparing premarket submissions for medical devices. This guidance document is intended to supplement FDA’s “Guidance for the Content of Premarket Submissions for Software Contained in Medical Devices” and “Guidance to Industry: Cybersecurity for Networked Medical Devices Containing Off-the-Shelf (OTS) Software”
FDA has published a draft of the guidance document that it has developed to assist industry by identifying issues related to cybersecurity that manufacturers should consider in preparing premarket submissions for medical devices. This guidance document is intended to supplement FDA’s “Guidance for the Content of Premarket Submissions for Software Contained in Medical Devices” and “Guidance to Industry: Cybersecurity for Networked Medical Devices Containing Off-the-Shelf (OTS) Software”