St. Jude Medical announced FDA approval of its next-generation Ellipse™ and SJM Assura™ portfolio of implantable cardioverter defibrillators (ICDs) and cardiac resynchronization therapy defibrillators (CRT-Ds). The new devices are designed to lower the risk of lead abrasion and to ensure high-voltage therapy delivery.
According to the announcement:
The Ellipse and SJM Assura family of devices feature the DynamicTx™ Over-Current Detection Algorithm, which automatically adjusts shocking configurations to ensure the delivery of high-voltage therapy even if an electrical short in one portion of the system were to occur. In addition, the next-generation Ellipse and SJM Assura portfolio of implantable defibrillators have a low-friction coating on the device can, which has been demonstrated in testing to significantly reduce the friction between the device and leads. As such, the low-friction coating provides an extra layer of insulation and is designed to reduce the risk for lead-to-can abrasion, the most common type of lead insulation failure in the industry.

 St. Jude Medical and privately-held Spinal Modulation, Inc., today announced that they have entered into a series of agreements under which St. Jude Medical made a $40 million equity investment in Spinal Modulation, a company that has developed an innovative neuromodulation therapy that provides a new pain management option for patients with chronic, intractable pain.
St. Jude Medical and privately-held Spinal Modulation, Inc., today announced that they have entered into a series of agreements under which St. Jude Medical made a $40 million equity investment in Spinal Modulation, a company that has developed an innovative neuromodulation therapy that provides a new pain management option for patients with chronic, intractable pain.
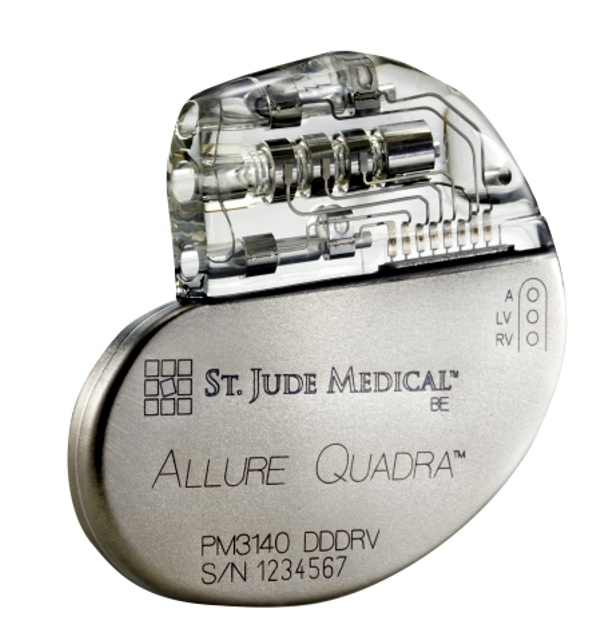 St. Jude Medical today announced CE Mark approval and European launch of its Allure Quadra™ Cardiac Resynchronization Therapy Pacemaker (CRT-P), which brings the quadripolar lead technology to the pacemaker market for the first time. According to the press release:
St. Jude Medical today announced CE Mark approval and European launch of its Allure Quadra™ Cardiac Resynchronization Therapy Pacemaker (CRT-P), which brings the quadripolar lead technology to the pacemaker market for the first time. According to the press release: