Image Credit: Boston Scientific
Boston Scientific has received CE Mark approval for increased longevity projections for the INCEPTA™, ENERGEN™, PUNCTUA™, COGNIS® and TELIGEN® implantable cardioverter defibrillators (ICDs) and cardiac resynchronization therapy defibrillators (CRT-Ds). The longevity projections are based on data submitted to the European authorities and vary for each device dependent on the model type and settings.
Projected device longevity exceeds 10 years for some models, approaches eight years for its CRT-D devices, and according to the press release is up to double that of comparable competitive device models. The company supports these devices with warranties of up to 10 years:
Warranties: INCEPTA and ENERGEN VR ICD: 10 years; INCEPTA and ENERGEN DR ICD: eight years; PUNCTUA and TELIGEN ICD: seven years; INCEPTA and ENERGEN CRT-D: six years; and PUNCTUA & COGNIS CRT-D: five years.

 I received today a
I received today a 
 Biotronik announced the European market release of BioMonitor®, an implantable cardiac device designed for the highly accurate and reliable monitoring and management of patients with atrial fibrillation (AF) or unexplained syncope.
Biotronik announced the European market release of BioMonitor®, an implantable cardiac device designed for the highly accurate and reliable monitoring and management of patients with atrial fibrillation (AF) or unexplained syncope.

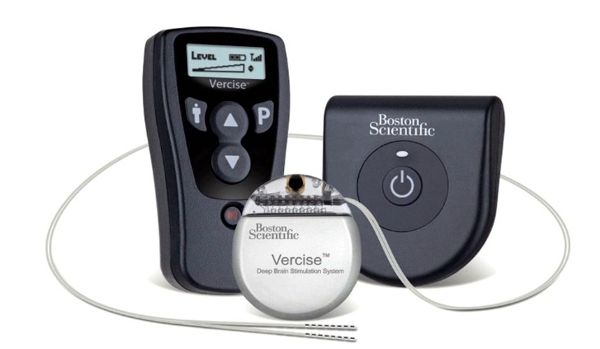


 St. Jude Medical announced it has received European CE Mark approval of its Eon™ family of neurostimulators for treating patients with intractable chronic migraine.
St. Jude Medical announced it has received European CE Mark approval of its Eon™ family of neurostimulators for treating patients with intractable chronic migraine.