
Swiss medical device manufacturer Sequana Medical developed the ALFApump® System to pump fluids accummulated in the abdomen of a patient into the bladder.

Swiss medical device manufacturer Sequana Medical developed the ALFApump® System to pump fluids accummulated in the abdomen of a patient into the bladder.

Image Source: Nevro's Website
Nevro Corp announced that FDA has granted approval for initiation of its SENZA-RCT study, a U.S. prospective, randomized, controlled pivotal clinical trial evaluating the safety and efficacy of Nevro’s high-frequency spinal cord stimulation system for the treatment of chronic pain.

Image Credit: Boston Scientific
Boston Scientific announced today that FDA has approved revised product labeling for its INCEPTA™, ENERGEN™, PUNCTUA™, COGNIS® and TELIGEN® ICDs and CRT-Ds to reflect increased longevity projections for these devices. The longevity projections are based on data submitted to the FDA and vary for each device dependent on the model type and settings.

Image Credit: St. Jude Medical
St. Jude today announced FDA approval of its Ellipse™ ICD. The device’s shape was designed with feedback from more than 200 physicians from around the world. The Ellipse ICD offers physicians unique design advancements, resulting in a high-energy ICD that occupies barely 30cc.
According to the announcement, “The Ellipse ICD’s unique shape was conceptualized by physicians during focus groups where they crafted in clay their vision for the ideal device design. The physician-inspired shape is unlike any device currently available and designed to increase patient comfort and physician ease-of-use. The angled header and rounded edges were designed to improve the way a lead wraps around the device once connected, which can result in a smaller incision and reduced pocket size for the device.”

Image Credit: St. Jude Medical
St. Jude Medical today announced FDA approval of its Assura™ portfolio of implantable cardioverter defibrillators (ICDs) and cardiac resynchronization therapy defibrillators (CRT-Ds).The new implantable defibrillators feature SecureSense™ RV Lead Noise Discrimination, an algorithm that expands the St. Jude Medical ShockGuard® Technology and offers advanced sensing options designed to reduce the incidence of inappropriate shocks for patients with these devices.
According to St. Jude’s announcement:
 Today EnteroMedics recorded revenue for the first time since it was incorporated nearly eight years ago. The company reported revenue of about $123,000 in the first quarter of the year from the sale of its Maestro RC implantable vagus nerve stimulation system for treating obesity. Revenue was generated through sales by its distribution partner in Australia.
Today EnteroMedics recorded revenue for the first time since it was incorporated nearly eight years ago. The company reported revenue of about $123,000 in the first quarter of the year from the sale of its Maestro RC implantable vagus nerve stimulation system for treating obesity. Revenue was generated through sales by its distribution partner in Australia.
This is an important step for an implantable device company that faced very tough times in 2009 after its US clinical trial failed to meet a critical effectiveness goal. EnteroMedics is currently conducting a pivotal trial that is expected to end in Q4 2012. EnteroMedics expects to file a premarket approval application with the FDA in the first half of 2013 if the data from the trial is positive.
 Yesterday Boston Scientific announced financial results for the first quarter ended March 31, 2012. Sales of Cardiac Rhythm Management devices were $501M vs. $559M for Q1 a year ago, or a decrease of 10%. Sales of Neuromodulation devices increased by 8% a year ago from $77M to $84M for Q1.
Yesterday Boston Scientific announced financial results for the first quarter ended March 31, 2012. Sales of Cardiac Rhythm Management devices were $501M vs. $559M for Q1 a year ago, or a decrease of 10%. Sales of Neuromodulation devices increased by 8% a year ago from $77M to $84M for Q1.
 St. Jude Medical today reported sales and net earnings for the first quarter ended March 31, 2012.
St. Jude Medical today reported sales and net earnings for the first quarter ended March 31, 2012.
Total CRM sales, which include ICD and pacemaker products, were $735 million for the first quarter of 2012, a 4 percent decrease compared with the first quarter of 2011. Of that total, ICD product sales were $450 million in the first quarter, a 3 percent decrease compared with the first quarter of 2011. First quarter pacemaker sales were $285 million, a decrease of 4 percent from the comparable quarter of 2011.
St. Jude Medical sales of neuromodulation products were $103 million in the first quarter of 2012, up 12 percent from the comparable quarter of 2011.

Image Credit: St. Jude Medical
Today St. Jude announced the first implant in its Accent MRI(R) Pacemaker and Tendril MRI(R) Lead IDE Study (MRI Study). The ultimate goal of the study is to determine if patients with these devices can safely undergo full-body, high resolution Magnetic Resonance Imaging (MRI) scans to better accommodate their medical needs. The investigational Accent MRI Pacemaker system from St. Jude Medical offers an advanced pacing platform that provides wireless telemetry and algorithms to help address individual patient conditions.
 In response to my post “A Challenge to History Buffs: Who Was Digikon?“, Paolo Pagani sent me the following message:
In response to my post “A Challenge to History Buffs: Who Was Digikon?“, Paolo Pagani sent me the following message:
“Digikon was in the years 1977-1985 the brand name product in Italy by Biotec Biomedical Technologies of Bologna – ITALY.
Pacemakers were a Digikon O.E.M. production for the trading company of Milan Italy KONTRON already a distributor in Italy of Medtronic.
Biotec developed the first pacemaker VVIR based on physiological changes in thoracic impedance due to respiration. (Biotec RDP-3)
Biotec-Bologna was acquired by Medtronic in August 1985.”
Thank you Paolo!
 Medtronic received FDA approval for the expanded use of CRT-D in mildly-symptomatic heart failure patients. The expanded indication includes New York Heart Association (NYHA) Class II heart failure patients with a left ventricular ejection fraction (LVEF) of less than or equal to 30 percent, left bundle branch block (LBBB), and a QRS duration greater than or equal to 130 milliseconds. Nearly 200,000 Americans are considered NYHA Class II, with another 620,000 people worldwide fitting this designation.
Medtronic received FDA approval for the expanded use of CRT-D in mildly-symptomatic heart failure patients. The expanded indication includes New York Heart Association (NYHA) Class II heart failure patients with a left ventricular ejection fraction (LVEF) of less than or equal to 30 percent, left bundle branch block (LBBB), and a QRS duration greater than or equal to 130 milliseconds. Nearly 200,000 Americans are considered NYHA Class II, with another 620,000 people worldwide fitting this designation.
The FDA’s decision for the expanded indication rests on data from the pivotal REVERSE (REsynchronization reVErses Remodeling in Systolic left vEntricular dysfunction) and landmark RAFT (Resynchronization/Defibrillation in Ambulatory Heart Failure Trial) clinical trials, which showed that CRT-D can benefit mildly symptomatic heart failure patients by reducing mortality and heart failure hospitalization rates.The expanded indication includes New York Heart Association (NYHA) Class II heart failure patients with a left ventricular ejection fraction (LVEF) of less than or equal to 30 percent, left bundle branch block (LBBB), and a QRS duration greater than or equal to 130 milliseconds. Nearly 200,000 Americans are considered NYHA Class II, with another 620,000 people worldwide fitting this designation.
Click here for press release from Medtronic.
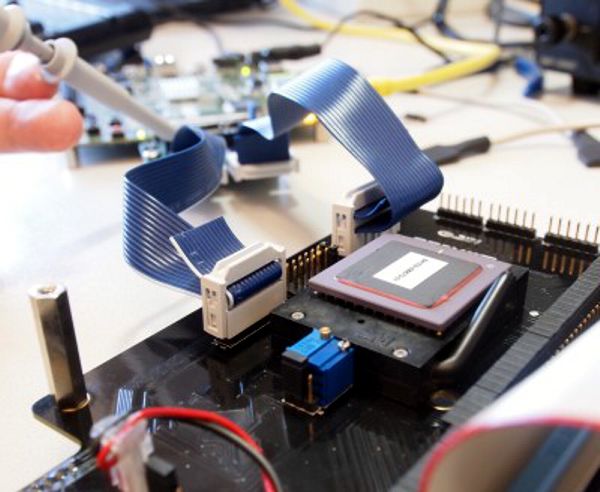
Image Credit: Monash Vision Group, Monash University, Australia
Engineers from the Monash Vision Group (MVG) have begun trialling the ASICs for a direct-to-brain visual prosthesis that is expected to enter human clinical trials in 2014.
The prosthesis will consist of a tiny camera mounted into a pair of glasses, which acts as the retina; a pocket processor, which takes the electronic information from the camera and converts it into signals enabling the brain to build up a visual construct; and cortical implants of several tiles which will be the portal for the stimulation of the visual cortex. Continue reading→
Image Credit: Fraunhofer Institute for Ceramic Technologies and Systems
Scientists at the Fraunhofer Institute for Ceramic Technologies and Systems developed a magnetically-coupled motor/generator system that they claim is able to transcutaneously transfer 100 mW to an implant up to 50 cm away.
In the external power-transfer module, a rotating magnet driven by an EC motor generates a magnetic rotary field. A magnetic pellet in the implanted receiver connects to the alternating exterior magnetic field and as a result, is set in rotation itself. The rotational movement is transformed into electricity, thus the power is produced right in the generator module. “With magnetic coupling, power can be transported through all non-magnetic materials, such as biological tissue, bones, organs, water, plastic or even a variety of metals. Moreover, the magnetic field produced has no harmful side effects on humans. It doesn‘t even heat up tissue,” says Dr. Holger Lausch, highlighting the advantages of the system. Continue reading→
 Johns Hopkins’ Sridevi V. Sarma, an assistant professor of biomedical engineering, has devised new seizure detection software that, in early testing, significantly cuts the number of unneeded brain-stimulation therapy that an epilepsy patient would receive.
Johns Hopkins’ Sridevi V. Sarma, an assistant professor of biomedical engineering, has devised new seizure detection software that, in early testing, significantly cuts the number of unneeded brain-stimulation therapy that an epilepsy patient would receive.
According to Sarma, “These devices use algorithms—a series of mathematical steps—to figure out when to administer the treatment,” Sarma said. “They’re very good at detecting when a seizure is about to happen, but they also produce lots of false positives, sometimes hundreds in one day. If you introduce electric current to the brain too often, we don’t know what the health impacts might be. Also, too many false alarms can shorten the life of the battery that powers the device, which must be replaced surgically.” Continue reading→