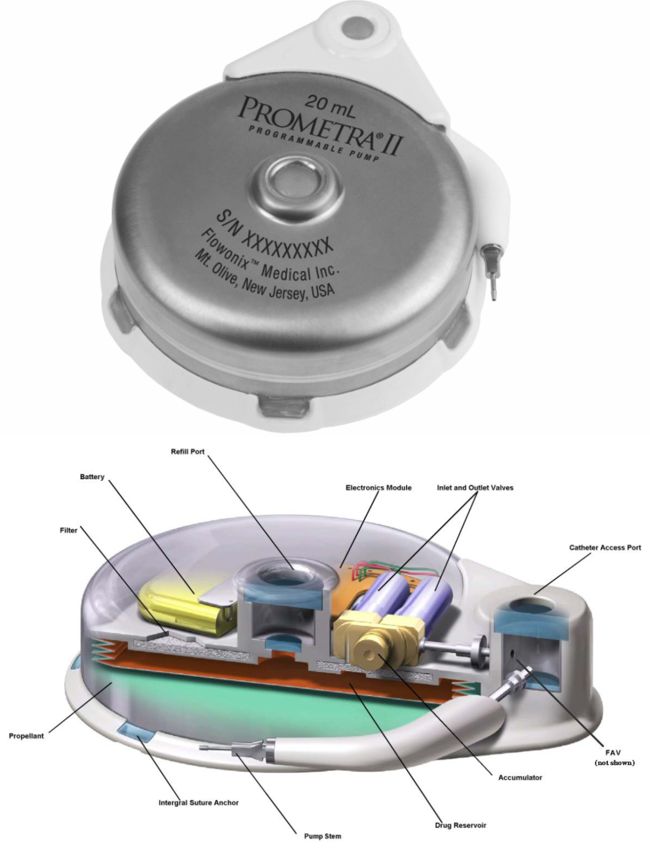
Image Credit: Flowonix Medical
Yesterday I was chatting with a friend regarding the medical device industry in NJ, and realized that I never posted a blog on Flowonix based in Mount Olive, NJ.
Flowonix was founded in 2005 (then called Medasys). It received approval to conduct its first clinical trial in 2007 on the Prometra programmable implantable pump. The company received approval by the FDA to market the Prometra in 2012 to deliver Infumorph.








 Impulse Dynamics, the company where I’m CTO and Executive VP,
Impulse Dynamics, the company where I’m CTO and Executive VP,