Yesterday, Jan 9, 2011, St. Jude Medical announced preliminary revenue results for the fourth quarter ended December 31, 2011:
“Fourth quarter cardiac rhythm management sales were approximately $728 million, a 4 percent decrease compared with the fourth quarter of 2010. Fourth quarter sales of implantable cardiac defibrillators were approximately $436 million, a 5 percent decrease from the comparable quarter in 2010. Pacemaker sales during the quarter were approximately $292 million, a 4 percent decrease compared with the fourth quarter of 2010.
…
Fourth quarter sales of neuromodulation products were approximately $121 million, a 12 percent increase compared to the fourth quarter of 2010.”
Company website: www.sjm.com


 I apologize for yesterday’s interruption of service in this blog. I moved hosting company because the old one was too slow and unreliable.
I apologize for yesterday’s interruption of service in this blog. I moved hosting company because the old one was too slow and unreliable.
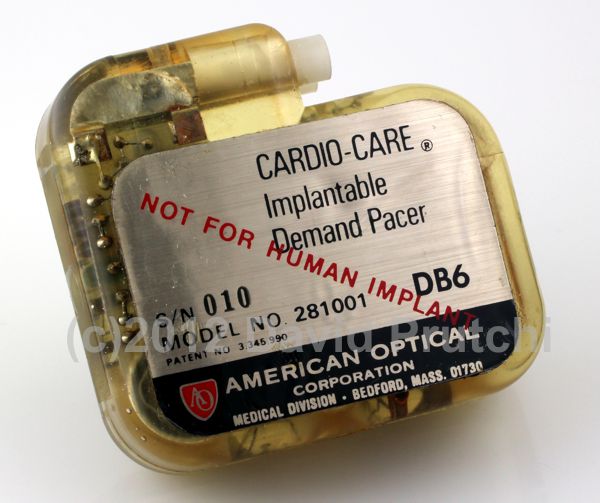


 Nanowattics was founded in 2007 to provide development services of ultra-low-power application-specific integrated circuits (ASICs) for safety-critical applications.
Nanowattics was founded in 2007 to provide development services of ultra-low-power application-specific integrated circuits (ASICs) for safety-critical applications.



 One of the indicators of metabolic demand that has been used for controlling the rate of pacemakers is central venous blood temperature (CVT).
One of the indicators of metabolic demand that has been used for controlling the rate of pacemakers is central venous blood temperature (CVT).



