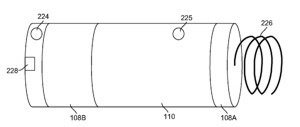
 Nanostim is an early-stage AIMD company in Milpitas, CA that is developing a pacemaker that can be implanted inside the heart through a catheter. The tiny device is attached directly to the heart, eliminating the need for leads.
Nanostim is an early-stage AIMD company in Milpitas, CA that is developing a pacemaker that can be implanted inside the heart through a catheter. The tiny device is attached directly to the heart, eliminating the need for leads.
In May 2011 Nanostim announced that St. Jude Medical had made a substantial investment in the company.
The company is operating in stealth mode, but some details about the leadless pacemaker have emerged from Nanostim’s patents and patent applications. An interesting detail is about the possible use of a betavoltaic power source: Continue reading



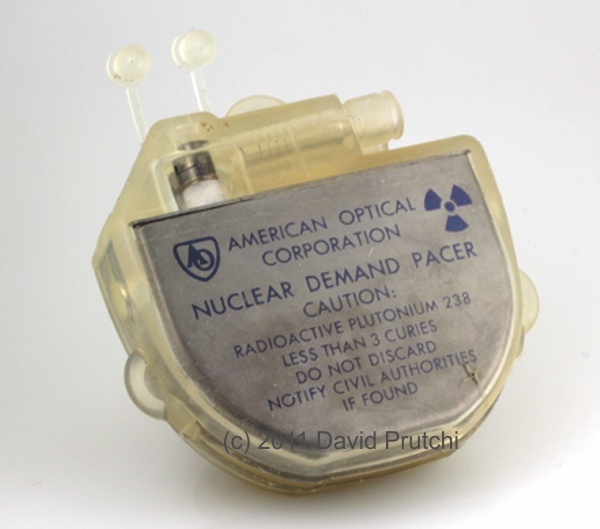


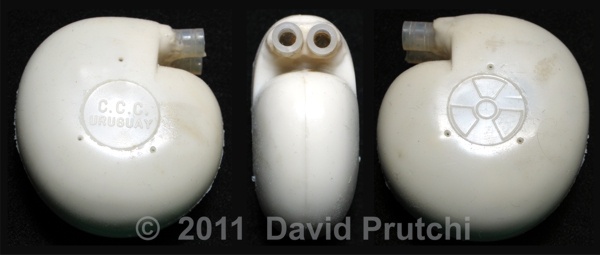



 CCC is one of the oldest pacemaker manufacturers in the world. It was founded in 1969 by Dr. Orestes Fiandra, who performed the first succesful, human, long-term pacemaker implant in the world.
CCC is one of the oldest pacemaker manufacturers in the world. It was founded in 1969 by Dr. Orestes Fiandra, who performed the first succesful, human, long-term pacemaker implant in the world.
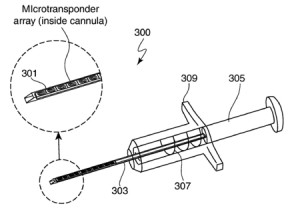

 Intrapace was founded in Mountain View, CA by
Intrapace was founded in Mountain View, CA by  Transneuronix, Inc. was founded in 1995 and was based in Mount Arlington, New Jersey. It was acquired by Medtronic in 2005.
Transneuronix, Inc. was founded in 1995 and was based in Mount Arlington, New Jersey. It was acquired by Medtronic in 2005.