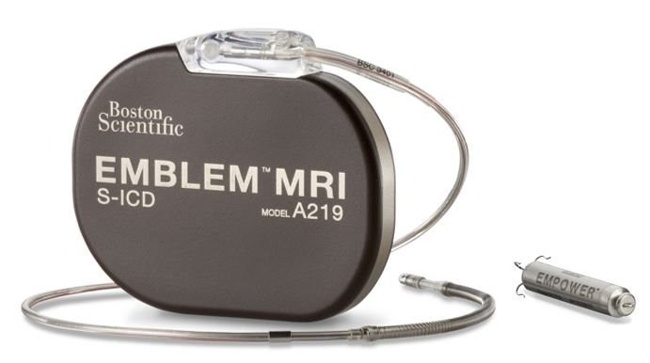
Image Credit: Intelligent Implants
Intelligent Implants is a company based in Cork, Ireland. Their SmartFuse® platform is a wirelessly enabled orthopedics platform that has been designed to remotely stimulate, control, and monitor bone growth. The goal of the SmartFuse system is to accelerate bone growth and provide remote monitoring of the patient to support real-time clinical decision-making. The first indication for the SmartFuse system will be for use in lumbar spinal fusions.
In an interview with MedGadget, Intelligent Implants’ CEO Ben Hertzog said about the implants’ remote monitoring functions:
“In addition to stimulation, we can also use the implant to measure the electrical properties of the surgical site. The electrical properties of the mature (healed) bone are quite different than that of the immediate post-operative surgical site, so by measuring these properties, over time, we can actually detect the new bone being formed and present this data to the surgeon in a way that is indicative of healing. Our SmartFuse Cloud system enables the physician to access the data in real-time. We can also track patient compliance and system status. Overall, we can provide the surgeon with an unprecedented view into the patient’s post-operative journey, including how well they are healing. We believe this ability to accelerate bone growth AND monitor healing and patient compliance has the potential to improve outcomes dramatically.”
FDA granted SmartFuse with Breakthrough Technology designation back in May of 2021.