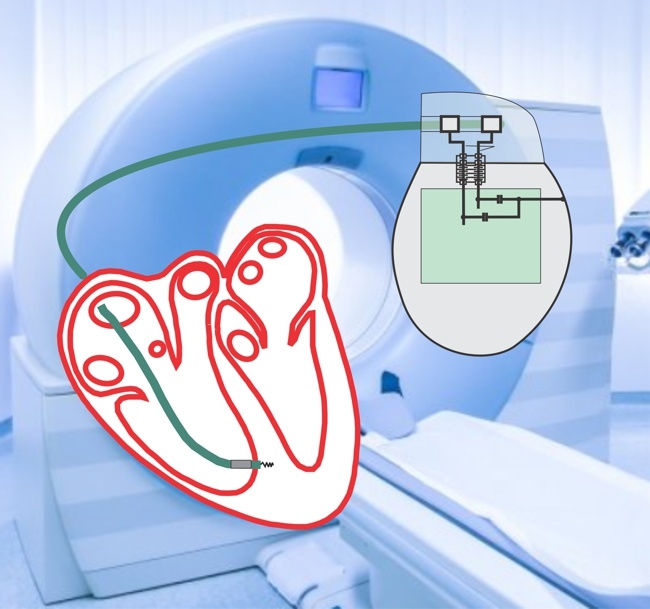
My colleagues and I presented yesterday two posters at the meeting of the International Society for Magnetic Resonance in Medicine (ISMRM). In these papers we provide support for the idea that MR-conditional leads from one system can be matched with MR-conditional IPGs from a different system without compromising safety related to RF-induced heating .
D. Prutchi, J. Meyers, R. Shehada, RF Impedance of MR-Conditional Pacemaker Leads when Connected to Implantable Pulse Generators from Different MR-Conditional Systems, ISMRM 2021, Poster 1701_2281, Paper
In this paper,we demonstrate that at 63.87 MHz the RF impedance of IPGs is minimal relative to that of the leads, which dominates the overall impedance of the implantable system. Accordingly, mixed hybrid systems composed of MR-Conditional leads and any MR-Conditional IPG are expected to have a comparable overall impedance and consequently produce the same RF-induced heating as their corresponding original systems specified by the manufacturers.
J. Meyers, D. Prutchi, R. Shehada, Input Impedance Comparison of MR-Conditional Cardiac Implantable Pulse Generators at the 1.5T MR Frequency of 63.87 MHz, ISMRM 2021, Poster 2311, Paper
In this study, we measured the impedance at 63.87 MHz of several MR-conditional IPGs from different manufacturers to assess the role of the IPG in determining the RF-induced heating at the lead electrodes. From the narrow impedance range measured, we suggest that the IPG component of an MR-Conditional system could be interchanged without compromising safety.
We also presented the following poster:
D. Prutchi, J. Meyers, R. Shehada, Importance of Pacemaker Lead Preconditioning for MR Safety In-Vitro Studies, ISMRM 2021, Poster 2282, Paper
In this study we investigated the change in the RF filtering characteristics of the leads of active implantable medical devices (AIMDs) as body fluids seep into the leads during the initial post implant period. Our findings indicate that the RF characteristics change dramatically with fluid absorption, making it necessary to precondition the leads by soaking in isotonic saline solution to simulate the in-vivo scenario when conducting in-vitro MR safety testing. Furthermore, leads designed with RF-attenuating lumped inductances must consider the effect of fluid absorption on changing the peak RF attenuation frequency.



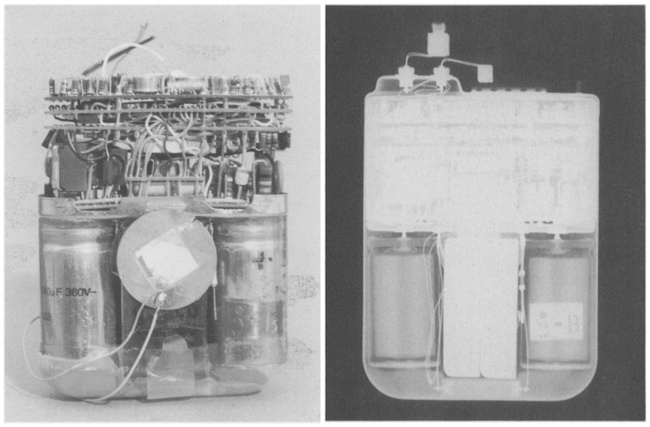

 Unlike Cyberonics’ VNS IPGs, the RNS® neurostimulator is designed to detect abnormal electrical activity in the brain and respond by delivering electrical stimulation to normalize brain activity before the patient experiences seizure symptoms. The neurostimulator is implanted in the cranium and connected to one or two leads that are implanted near the patient’s seizure focus.
Unlike Cyberonics’ VNS IPGs, the RNS® neurostimulator is designed to detect abnormal electrical activity in the brain and respond by delivering electrical stimulation to normalize brain activity before the patient experiences seizure symptoms. The neurostimulator is implanted in the cranium and connected to one or two leads that are implanted near the patient’s seizure focus.